Novel Titanium Dioxide Catalyst Support for Electrocatalytic Carbon Dioxide Reduction
Researchers from Japan propose an in-liquid plasma-treated titanium dioxide catalyst support decorated with silver nanoparticles as an alternative to carbon catalyst for effective carbon dioxide reduction into valuable resources.
A chemically stable titanium dioxide electrode decorated with silver nanoparticles shows promise for converting carbon dioxide reduction into useful resources
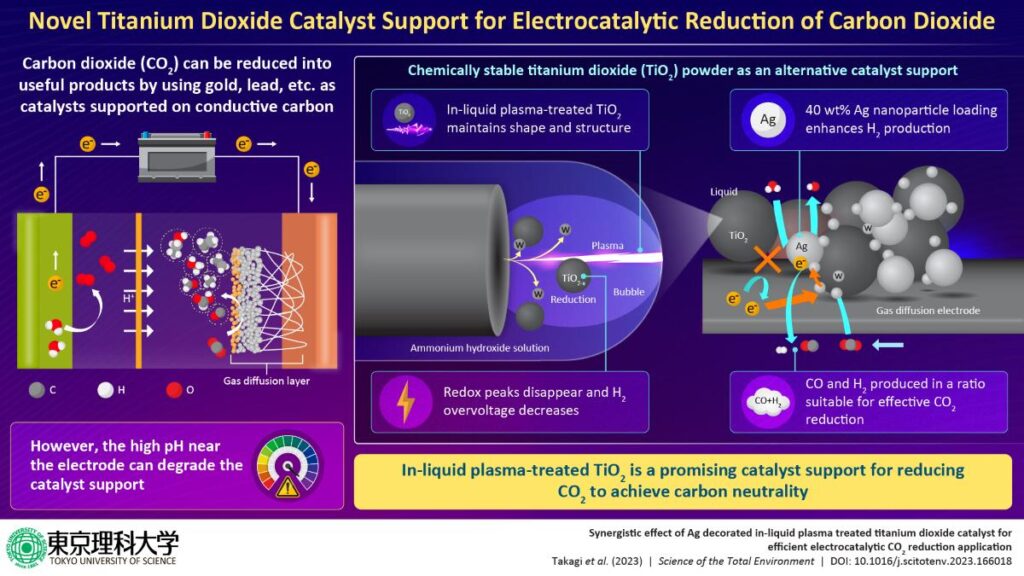
Carbon dioxide can be electrocatalytically reduced to useful resources using conventional catalysts such as gold or lead supported on conductive carbon. However, the high pH environment near electrodes often degrades the catalyst support, rendering them ineffective. Now, researchers have developed novel in-liquid plasma-treated titanium dioxide electrode decorated with silver nanoparticles as an alternate catalyst support, facilitating enhanced conversion of carbon dioxide to useful products, such as syngas, a clean alternative to fossil fuels.
The conversion of atmospheric carbon dioxide (CO2), a greenhouse gas, to useful resources such as carbon monoxide, formic acid, and methanol and their byproducts is considered a promising route to mitigating global warming as well as generating economic value. One approach to CO2 conversion is through electrocatalytic reduction. This process utilizes conventional catalysts, such as lead, silver, tin, copper, gold etc. supported on conductive carbon as electrode material for selectively CO2 reduction. However, the electrode is often exposed to a high pH environment of the electrolyte during electrocatalysis, which can degrade the catalyst support and is a cause of major concern.
To address this challenge, a team of researchers, led by Mr. Kai Takagi and Prof. Chiaki Terashima from Graduate School of Science and Technology and Research Institute for Science and Technology at Tokyo University of Science (TUS) in Japan, has recently developed a catalyst support based on titanium dioxide (TiO2) powder, a compound commonly used in sunscreen, paints, coatings, toothpaste, plastics, paper, pharmaceuticals, and food coloring, as an alternative to carbon for facilitating effective CO2 reduction. Their work was made available online on 4 August 2023 and published in Volume 902 of the journal Science of the Total Environment on 1 December 2023.
The researchers first carried out surface treatment using safe and inexpensive in-liquid plasma to improve the electrochemical properties of TiO2. “The in-liquid plasma-treated TiO2 maintained its particle shape and crystal structure. Additionally, elemental analysis and evaluation of the interfacial bonding state and electrochemical properties of TiO2 revealed that the redox peaks corresponding to Ti4+ and Ti3+ derived from TiO2 disappeared and the hydrogen overvoltage decreased,” highlights Prof. Terashima. These observations led the team to conclude that tungsten coating or doping occurred on some portions of the reduced TiO2 surface.
The researchers then used the TiO2 as a carrier and loaded it with silver nanoparticles (AgNPs), which act as catalysts, to develop a gas diffusion electrode for CO2 reduction. While untreated TiO2 exhibited high selectivity for CO2 and carbon black, in-liquid plasma-treated TiO2 with 40 wt% AgNP loading demonstrated increased hydrogen production and enhanced catalytic performance. Given that a suitable ratio of hydrogen to carbon monoxide is important for effective CO2 reduction, the presented technology, thus, has tremendous potential for converting CO2 to useful byproducts, such as syngas, which is considered a clean fuel with very high industrial value.
Additionally, the electrocatalytic reduction of CO2 can be integrated with renewable energy sources, such as solar panels or wind power, for sustainable and environmentally friendly CO2 conversion. Therefore, this work is a significant step towards efficiently tackling greenhouse gas emissions and fighting climate change.
“Hopefully, the present study will promote research on technologies for carbon neutrality and carbon recycling, in alignment with the United Nations Sustainable Development Goals 7, 12, and 13 on affordable and clean energy, responsible consumption and production, and climate action, respectively. These, in turn, will open doors to the realization of a carbon-neutral and sustainable future,” concludes Prof. Terashima.
And we hope his vision is realized soon!
Reference
Title of original paper: Synergistic effect of Ag decorated in-liquid plasma treated titanium dioxide catalyst for efficient electrocatalytic CO2 reduction application
Journal: Science of the Total Environment
DOI: https://doi.org/10.1016/j.scitotenv.2023.166018
About The Tokyo University of Science

Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan’s development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of “Creating science and technology for the harmonious development of nature, human beings, and society,” TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today’s most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
Website: https://www.tus.ac.jp/en/mediarelations/
About Professor Chiaki Terashima from Tokyo University of Science
Prof. Chiaki Terashima obtained an undergrad degree in Science and Engineering in 1994 from the Tokyo University of Science, and a PhD from the Division of Engineering of the University of Tokyo in 2003. He has been with Tokyo University of Science since 2013, where he conducts research on plasma chemistry and diamond materials. He is now a member of the Carbon Value Research Center as well as the Research Center for Space System Innovation, both at the Tokyo University of Science.
For more information, please visit: https://www.tus.ac.jp/en/fac/p/index.php?673c
Funding information
This study was supported by the Program on Open Innovation Platform with Enterprises, Research Institute and Academia (OPERA, grant numbers JPMJOP1843) of the Japan Science and Technology (JST).
Media contact
Hiroshi Matsuda
Public Relations Division, Tokyo University of Science
Email: mediaoffice@admin.tus.ac.jp