Bright China 2025: Airdoc PBM Vision Rehabilitation Device Clinical Research Data Release


HONG KONG, Nov 11, 2025 – (ACN Newswire) – Recently, the Bright China 2025 Clear Vision China Myopia Prevention and Control Conference & International Myopia Symposium was held in Shanghai. During the conference, on October 26, Airdoc jointly organized a satellite session themed “Clinical Research and Prospects of PBM(R) Photobiomodulation” with several authoritative experts. At the session, clinical research data on the application of Airdoc’s PBM(R) Vision Rehabilitation Device was released, with the aim of promoting the high-quality development of myopia prevention and control endeavors and assisting in achieving the national strategic goals for myopia prevention and control health.
At the “Lighthouse – Standards and Guidelines” thematic seminar, Professor Zou Haidong from the Shanghai Eye Disease Prevention and Treatment Center introduced the clinical trial progress of the Airdoc PBM(R) Vision Rehabilitation Device. Professor Zou mentioned, “The currently ongoing clinical trial data shows that, compared with a placebo, the application of the Airdoc PBM(R) Vision Rehabilitation Device can effectively control the myopic shift in axial length and refractive power.”

Meanwhile, the satellite session themed “Clinical Research and Prospects of PBM(R) Photobiomodulation” was jointly chaired by Professor Wang Xiaojuan from Shanghai First People’s Hospital, Shanghai Jiao Tong University School of Medicine, and Professor Yu Jun from Xinhua Hospital, Shanghai Jiao Tong University School of Medicine. At the session, Professor He Xiangui from the Shanghai Eye Disease Prevention and Treatment Center, Professor Liu Hong from Shanghai Children’s Medical Center, and Professor Chen Zhijun from Children’s Hospital of Nanjing Medical University delivered wonderful thematic reports, providing in-depth analyses of the groundbreaking progress of PBM photobiomodulation technology in the field of myopia prevention and control.

Bright China 2025 | Moderators of Airdoc’s Satellite Session: Professor Wang Xiaojuan (Left) and Professor Yu Jun (Right)
Clinical Research Data on the Solo Application of Airdoc PBM(R) Vision Rehabilitation Device
Professor He Xiangui from the Shanghai Eye Disease Prevention and Treatment Center pointed out that although there are currently multiple myopia prevention and control methods in clinical practice, significant individual differences exist among children and adolescents. Some children respond poorly to conventional intervention measures, and there is an urgent need to explore more precise and personalized supplementary solutions. Against this backdrop, PBM photobiomodulation technology, with its advantages of being non-invasive, highly safe, and highly synergistic, has become a research hotspot and an important option in clinical practice.

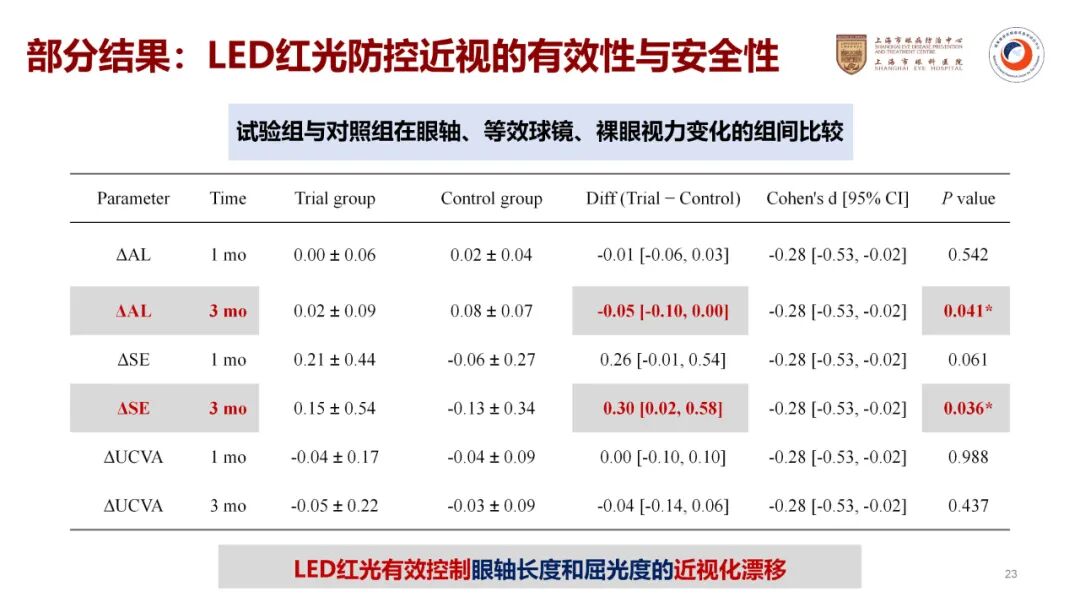
The Shanghai Eye Disease Prevention and Treatment Center conducted a single-center, randomized controlled pilot study titled “LED Red Light for Controlling Myopia Progression.” The study included 40 children aged 8 to 12 with simple myopia, who were divided into an intervention group and a control group, with 20 participants in each group. The intervention group received irradiation from the Airdoc PBM(R) Vision Rehabilitation Device (2 sessions per day, 3 minutes per session), while the control group was subjected to extremely low-dose irradiation (0.001 mW, simulating a placebo).
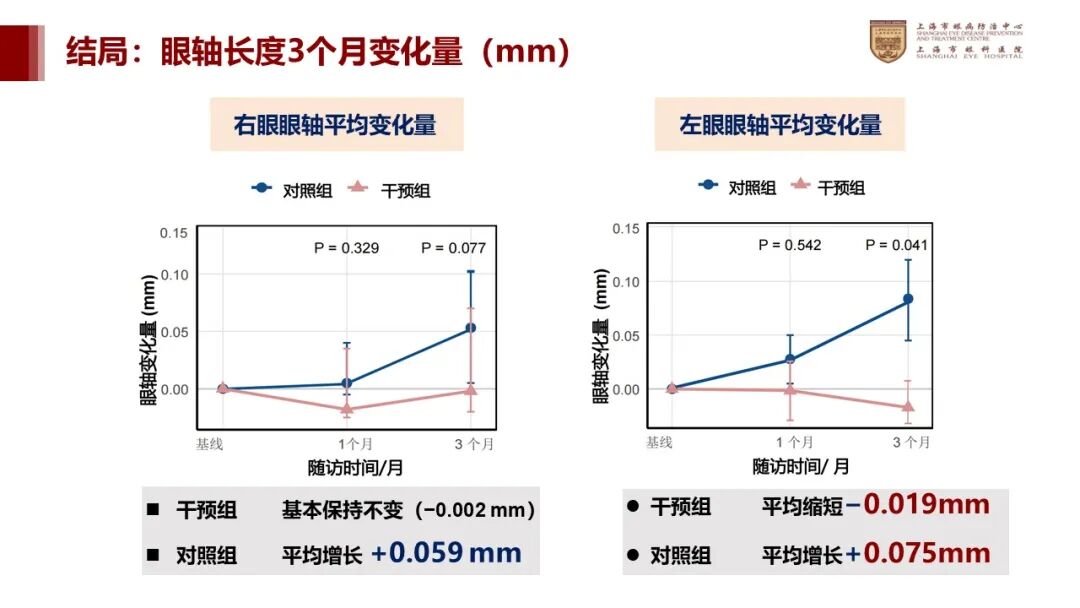
The pilot study data revealed that, over a 3-month period, the average axial length changes in the intervention group were as follows: right eye, -0.002 mm; left eye, -0.019 mm. In the control group, the axial length changes were: right eye, +0.059 mm; left eye, +0.075 mm.
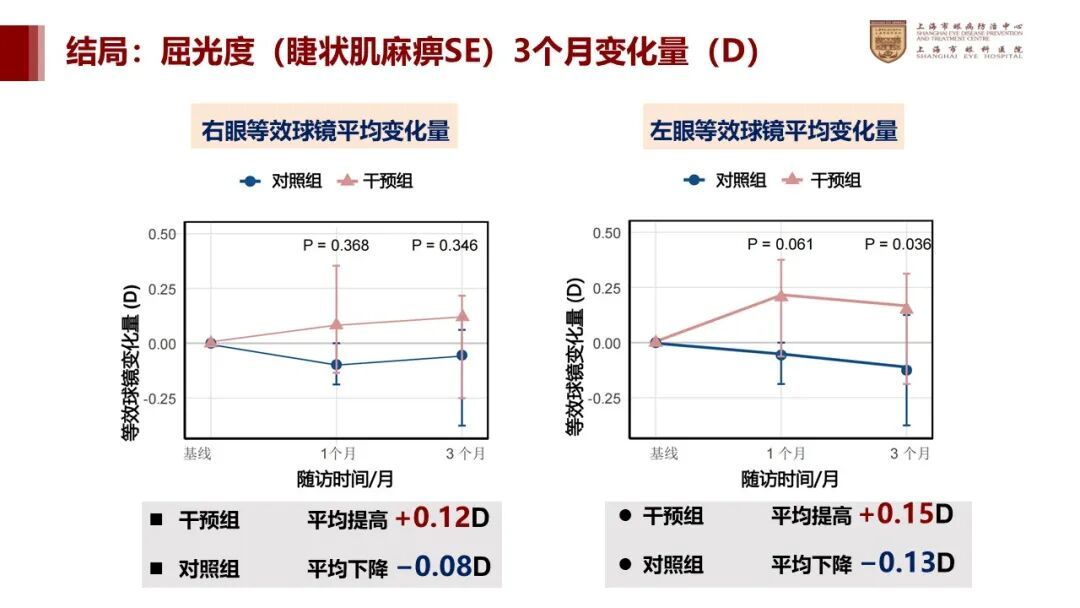
The change in diopters over the 3-month period for the intervention group was: +0.12D for the right eye and +0.15D for the left eye. For the control group, the average change was: -0.08D for the right eye and -0.13D for the left eye.
Compared with the control group, the myopic shift in both axial length and diopter was significantly slowed down in the intervention group. Moreover, up to 85% of participants in the intervention group maintained or improved their vision, which was higher than the 50% in the control group, indicating a higher rate of visual stability. Additionally, there were no serious adverse events, and the compliance was good.
Synergistic Enhancement Effect Achieved by Combining Airdoc PBM(R) Vision Rehabilitation Device with Defocus Lenses
Professor Liu Hong from Shanghai Children’s Medical Center pointed out in her report titled “Research on the Application of Photobiomodulation in Myopia Control” that myopia prevention and control pose a significant challenge worldwide, and ensuring both safety and efficacy is of utmost importance.
Currently, the PBM technology applied for myopia prevention and control in China has undergone five iterations. The LED light source technology has also evolved from small LED light spots to the globally advanced PBM-LED(R) annular light spot technology [1]. This technology not only avoids the foveal region of the retina’s macula but also safeguards children’s clear vision in a safer and more effective manner.

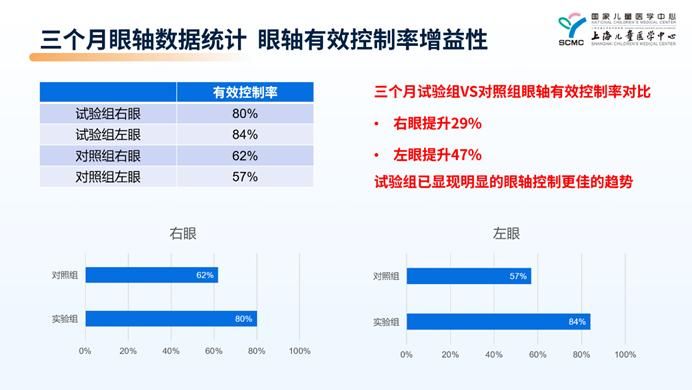
Professor Liu Hong presented the preliminary clinical data from a prospective randomized controlled clinical trial currently underway at Shanghai Children’s Medical Center. The results demonstrated that after three months of treatment combining defocus lenses with the Airdoc PBM(R) Vision Rehabilitation Device, the effective control rates of axial length elongation reached 80% for the right eye and 84% for the left eye. Compared to using defocus lenses alone, this represented a 29% improvement for the right eye and a 47% improvement for the left eye. The preliminary findings indicate that a combined myopia prevention and control approach can achieve a “synergistic enhancement” effect in children and adolescents.
PBM Targeting ipRGC Cells: Unlocking New Keys to Myopia Prevention and Control from the Perspective of Brain Science
Professor Chen Zhijun from Children’s Hospital Affiliated to Nanjing Medical University stated, “PBM targeting ipRGC cells regulates the eye-brain signaling, breaking through the boundaries of traditional optical interventions and providing a new pathway for preventing myopia in children.” Myopia is not merely a refractive issue but also a typical representative of “eye-brain axis” disorders. Recent studies have found that photobiomodulation, acting on the intrinsically photosensitive retinal ganglion cells (ipRGCs), may delay axial length elongation through mechanisms such as regulating dopaminergic signaling pathways, circadian rhythm expression, and pupillary light reflex.

The impact of PBM photobiomodulation technology on the visual signal transduction network offers new insights for breaking through the traditional single-optical correction model. Future myopia prevention and control necessitate the establishment of a multidimensional integrated treatment system, driving innovation in personalized and precision-based myopia prevention strategies.
Looking ahead, Airdoc will continue to fulfill its mission of “making health accessible everywhere,” continuously exploring and innovating in the field of AI-driven myopia prevention and control. We aspire to collaborate with more ecosystem partners to build a globally leading AI-integrated diagnosis and treatment solution for myopia prevention and control, advancing the standardization and widespread adoption of PBM(R) non-invasive phototherapy technology. Together, we aim to illuminate a clear vision for children and adolescents, co-creating a brilliant future for their eye health!
Reference:
[1] National Intellectual Property Administration – Patent No. ZL202410456292.3
Note: PBM(R) and PBM-LED(R) are trademarks legally registered by Airdoc Technology and are protected by relevant laws and regulations, including the “Trademark Law of the People’s Republic of China.” Airdoc Technology legally enjoys the intellectual property rights associated with these trademarks. Without authorization, no entity or individual shall use them.
Introduction to Airdoc
Founded in 2015, Airdoc is committed to ” More Intelligence,Better Care” providing comprehensive artificial intelligence (AI) solutions for early screening and management of chronic diseases, myopia prevention and control, treatment of strabismus and amblyopia, and stress resilience assessment. As a global leader in the field of retinal image AI, Airdoc is also the first enterprise in China to obtain Class III medical device registration for AI-assisted diagnosis of fundus diseases from the National Medical Products Administration (NMPA).
In November 2021, Airdoc successfully went public on the Hong Kong Stock Exchange, becoming the “first medical AI stock.” Airdoc possesses core technologies in deep learning algorithms and ranks among the top globally in terms of retinal technology patents. It has been honored with the highest award in China’s AI field, the “Wu Wenjun AI Technological Progress Award,” as well as the “Beijing Science and Technology Progress Award.” Currently, Airdoc’s AI-powered retinal imaging products are widely applied in various settings, including graded hospitals, community clinics, physical examination centers, insurance companies, optometry centers, and pharmacies, providing disease-assisted diagnosis and health risk assessments to tens of millions of users.
Copyright 2025 ACN Newswire. All rights reserved. www.acnnewswire.com