Cyrus Biotechnology’s COVID therapeutic lead, ACE2.v2.4, strongly binds and neutralizes SARS-CoV-2 omicron variant potentially providing long term protection

Therapeutic was designed and engineered to provide broad variant coverage, with the potential to offer COVID treatment for years to come. Current approved biologic drugs and clinical candidates show reduced or drastically reduced potency.
SEATTLE–(BUSINESS WIRE)–Cyrus Biotechnology, Inc., a Seattle-based biotechnology firm with a proprietary platform for biologics discovery that combines software, AI and large-scale parallel protein screening, today announced that its pre-clinical stage COVID therapeutic lead molecule, ACE2.v2.4 strongly binds the spike protein of SARS-CoV-2 variant of concern (VOC) omicron. ACE2.v2.4 was engineered to bind to future variants of the virus, providing a therapeutic for COVID for years to come, and these data demonstrate that molecule is capable of providing superior protection against novel variants.
ACE2.v2.4, a soluble derivative of the natural receptor for the virus, was specifically designed in collaboration with researchers at the University of Illinois to tolerate mutations in the spike protein of the virus, and also has high affinity for the alpha and delta VOCs as well as for SARS-CoV-1 and for a set of unrelated bat coronaviruses (reported in Science Advances).
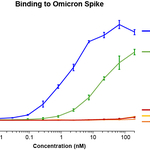
Testing from multiple laboratories, including at Cyrus, has shown that antibodies currently on the market – a cocktail from Regeneron and antibodies from Vir/GSK and from Eli Lilly – have lost some or all of their efficacy against omicron and other variants. In contrast, new work, performed at Cyrus and the University of Illinois, now shows that ACE2.v2.4 retains nearly all of its efficacy against omicron. These data are shown in the figure included alongside this press release, and confirm antibody results seen by others such as the Diamond group. ACE2.v2.4 has already been shown to tightly bind every VOC in cell-based assays and to neutralize the original virus in mouse studies. Recently released pseudovirus neutralization studies from the Hoshino laboratory in Kyoto confirms that Cyrus’s ACE2.v2.4 (and other Cyrus variants) successfully neutralize omicron.
“These are early data in a developing program, and we still need to see clinical performance, but Cyrus Biotechnology’s ‘ACE.v2’ SARS-CoV-2 decoy receptor — which was developed against the original Dec 2019 Spike — remains smashingly effective at neutralizing Omicron and other variants”, said Dr. Jeremy Kamil, associate professor of microbiology and immunology at Louisiana State University Health Shreveport.
“New COVID-19 variants continue to emerge and surprise us by escaping monoclonal antibody cocktails and vaccines. So called ‘decoys’ like ACEv2.4 represent exciting therapeutic candidates. Because ACE2.v2.4 mimics yet greatly improves upon how the human ACE2 receptor binds the viral spike protein, these candidate therapeutics work quite differently from monoclonal antibodies. So, the real promise here is that such interventions may be much harder for the virus to escape.”
Cyrus is pursuing confirmatory studies in animal models of COVID-19 and is continuing to improve pharmacokinetic performance of the lead molecule. Other related molecules with even higher potency against omicron and earlier SARS-COV-2 variants are also under evaluation. Initial development of ACE2.v2.4 has been published and newer data showing efficacy against VOCs has been accepted in a peer-reviewed journal. Cyrus will continue to expeditiously publish data on omicron and beyond.
ACE2.v2.4 is a dual-mode-of-action COVID-19 therapeutic lead molecule with virus neutralizing and anti-inflammatory/lung-protective enzymatic activity. “Many groups have created therapeutics that bind to the virus spike protein and prevent binding and entry of the virus into human cells”, said Dr. Erik Procko, Director of Discovery at Cyrus and Associate Professor of Biophysics at the University of Illinois. “The antibody therapies create new proteins to bind the spike, mimicking natural ACE2 binding. Cyrus took a different approach, starting from the natural ACE2 and improving it to have potency like antibody. By engineering ACE2.v2.4 to be as similar to natural ACE2 as possible, the ACE2.v2.4 is engineered to bind to any SARS-CoV-2 variant. New CoV-2 variants that do not bind ACE2.v2.4 will also not bind human cells and these variants will not be infective. Based on this engineering method, and results with delta and omicron and other variants, we believe that ACE2.v2.4 has a much better chance of neutralizing future VOCs than any antibody or cocktail of antibodies,” Dr. Procko added.
“While new therapeutic antibodies can be generated over a period of months as new VOCs arise, a broadly active protein built as a decoy for the normal ACE2 receptor should provide an intervention that remains effective and clinically useful for years and perhaps indefinitely for endemic COVID-19,” said Cyrus CEO Lucas Nivon.
About Cyrus Biotechnology
Cyrus Biotechnology is a pre-clinical-stage biotech company combining computational and experimental protein design and screening to create novel biologics for serious unmet medical needs. Using this approach, Cyrus is developing an early pipeline of innovative programs in multiple indications. The Cyrus platform improves both the efficacy (binding affinity, aggregation propensity, solubility, and stability) and safety (binding specificity and immunogenicity) of natural proteins. Cyrus is also partnering with leading biotech and pharma companies and research institutes to bring collaborative programs forward from discovery to the clinic. Cyrus is based on core software from the lab of David Baker at the University of Washington. Cyrus has worked with over 90 industry partners. We are based in Seattle, WA and financed by leading US and Asian Biotech and Tech investors including Orbimed, Trinity Ventures, Springrock, Agent Capital, iSelect, Yard Ventures, WRF, and Alexandria. For more information about Cyrus please visit https://cyrusbio.com/
NOTICE: The information contained in this document is dated as of December 21, 2021. Cyrus Biotechnology, Inc. (the Company) disclaims any obligation to update such information after such date. This document contains forward–looking statements reflecting the Company’s current expectations that necessarily involve risks and uncertainties. Actual results and the timing of events may differ materially from those contained in such forward-looking statements due to a number of factors and the Company undertakes no obligation to revise or update any forward-looking statement to reflect events or circumstances after the issuance of this press release.
Contacts
Lucas Nivon
lucas@cyrusbio.com
206-258-6561