KSL Diagnostics, Inc. Launches First-of-its-Kind Antibody Test That Measures Degree of Immunity from COVID-19

COVID-19 Immune Index™ Can Monitor the Level of Virus Protection
BUFFALO, N.Y.–(BUSINESS WIRE)–#antibodytest–KSL Diagnostics, Inc. (“KSL”), developers of novel diagnostic and therapeutic applications for immunology and oncology, has launched a first-of-its-kind antibody test that detects an individual’s immune response to COVID-19 and assesses the risk of infection if subsequently exposed. The COVID-19 Immune Index™ can help monitor effectiveness of COVID-19 virus protection through a simple blood test.
This new assay correlates COVID-19 virus neutralization against a person’s antibody levels. Recognizing that people have a need to clearly understand their level of risk for COVID-19 infection and potential complications, KSL designed and developed a new, highly accurate test that measures antibodies specific to COVID-19 which develop in response to vaccination or infection and correlates these results with virus neutralization studies incorporating the significant COVID-19 virus strains to date.
“There is a lot of variability in antibody levels over time after infection, vaccination and boosters. Guidance is constantly changing, but good tools to help people better understand their immune status have not been available,” says Kevin Lawson, President and CEO of KSL. “The COVID-19 Immune Index™ provides an accurate report for those who are immunocompromised, at risk with co-morbidities, re-entering the workplace or traveling. Understanding your level of immunity can provide peace of mind as we move past the pandemic.”
The new test provides physicians and their patients an objective datapoint to help determine appropriate timing for booster vaccine doses and making informed decisions related to potential COVID-19 exposure. The test will be valuable for monitoring the immunity levels of nursing home and long-term care residents, veterans living in group housing, and patients undergoing treatment for cancer, organ transplants, or serious chronic illnesses, among others. KSL’s COVID-19 Immune Index™ is approved by New York State Department of Health/CLIA/Emergency Use Authorization (EUA).
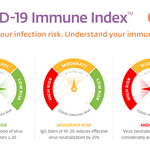
In collaboration with the University at Buffalo Center for Advanced Technology in Big Data and Health Sciences (“UB CAT”) and Dr. Amy Jacobs, a researcher leading UB’s Jacobs School of Medicine and Biomedical Sciences’ Biosafety Containment Level 3 facility—required to handle highly infectious viral pathogens like SARS-CoV-2—KSL completed studies on the relevance of circulating antibodies in vaccinated individuals. The aim was to determine the optimal neutralizing antibody titers required to block virus entry into host cells by utilizing a plaque reduction neutralization test (PRNT), the “gold-standard” for assessing virus deactivation and correlating these results with KSL’s antibody assays. Results demonstrated stratification of immunity. For example, 100% neutralization of the virus was seen at IgG levels of 20 and above, suggesting adequate immunity. IgG levels from 10-20 reduced effective virus neutralization by 25%, while IgG levels below 10 indicated considerably decreased neutralization, suggesting ineffective immunity.
“The ability to correlate COVID-19 neutralizing antibody titers, especially IgG titers, with antibody assays to accurately assess an individual’s level of protection from COVID over time is a breakthrough development that will help us better navigate the pandemic as it transitions to endemic,” says Dr. Amy Jacobs of the University at Buffalo. “Because KSL’s COVID-19 Immune Index™ test compares well with the industry-standard PRNT assay but is better suited to high throughput testing, physicians will be able to conduct large scale community testing to monitor declining immunity in specific populations and actively promote boosters to curb new surges of the disease.”
“UB CAT, a program of Empire State Development’s Division of Science, Technology and Innovation (NYSTAR), supports university-industry collaborations to accelerate innovative ideas to market. The success of this project is a testimony to the strength of partnerships between university experts, industry partners and New York State resources to quickly make new commercial products available—in this case, to help people navigate the continued uncertainties of COVID-19,” says Smitha James, Associate Director for UB CAT.
KSL provides COVID-19 Immune Index™ testing as a service. Tests can be ordered at www.immuneindex.com or www.ksldx.com. In addition to the COVID-19 Immune Index™, KSL offers a comprehensive range of COVID-19 testing as well as broad diagnostic services for immunology, infectious diseases, and oncology. More information, including appointment scheduling, pricing, and testing locations, can be found at: https://www.ksldx.com.
About KSL Diagnostics Inc.
KSL (www.ksldx.com) operates New York State Department of Health / CLIA certified clinical laboratories licensed throughout the US, including Beutner Laboratories and the Robert Guthrie Laboratory. The rapidly expanding menu integrates standard of care tests and novel assays, developed at KSL and through partnership with innovative diagnostic companies. KSL provides superlative regulatory compliance and industry-leading standards of service.
For more information, visit www.ksldx.com.
Contacts
Mindy Eras, Cheryl Lechok
GYC Vegas
mindy@gycvegas.com
cheryl@gycvegas.com